1.1 Avian Migration
During migration, birds display an ability to travel during day and night, across large areas of featureless ocean or desert, correcting course when displaced [1].
from Wikipedia
This indicates that they are not only using visual and olfactory markers to form a map, but also have an internal compass by which they are able to determine their orientation, thus allowing them to perform true navigation [2].
Experiment reveals that birds are sensitive to magnetic fields on the order of the Earth’s magnetic field (50 µT) [3], supporting the hypothesis that they are detecting and using this field during migration.
Their method of detection also seems to require light, as their use of a magnetic compass in course correction is inhibited at night [4].
The Earth’s magnetic field does not usually have an impact on biological systems because it is so weak: the effect of thermal fluctuations is far stronger than any interactions between organic matter and the field. This raises the question of how birds could be using this field to navigate, as they do not contain high levels of ferromagnetic materials that would facilitate magnetoreception [5].
1.2 The Radical Pair Mechanism
While a weak field is unlikely to have an effect on its own, it might bias the final state of a system far from equilibrium, where there are several equally possible outcomes.
Consider the analogy of a heavy block about to fall over. Even a small force, such a fly landing on one side, might make the rock fall in one direction or the other, moving it much more than it would be able to if the rock were stable.
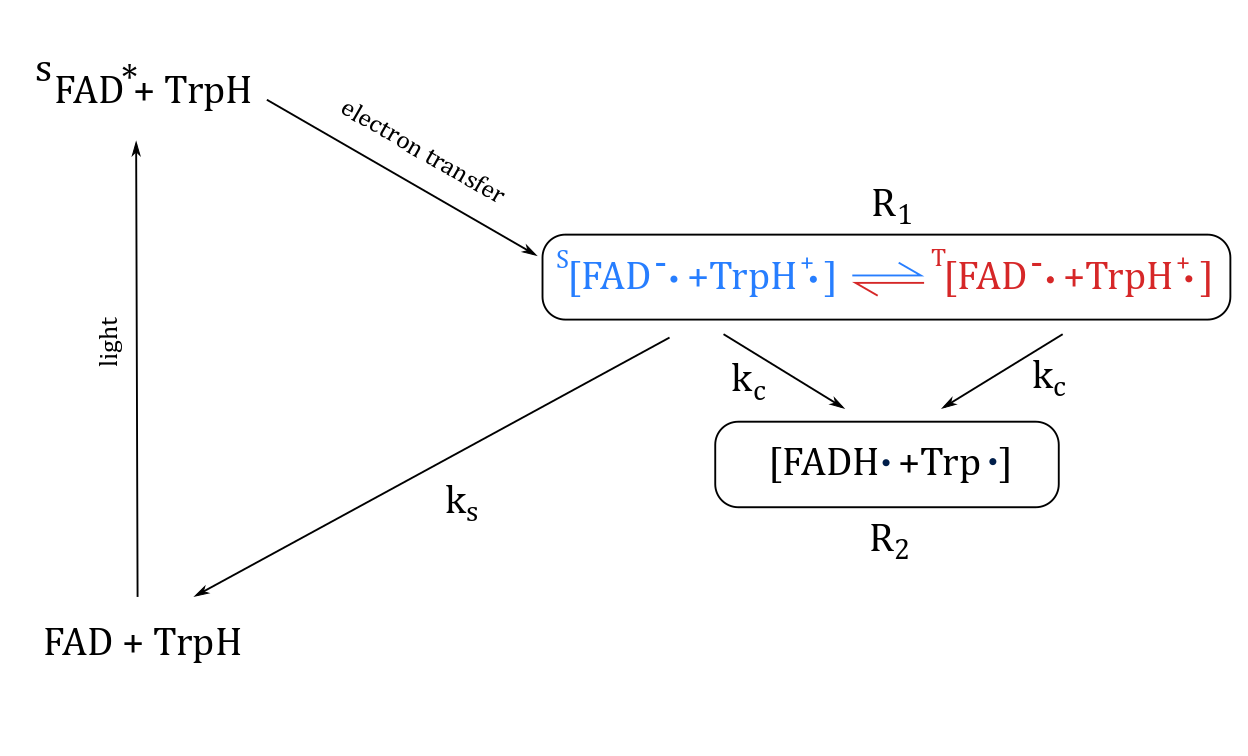
Schulten et al. first proposed a chemical mechanism for avian magnetoreception in 1978, in which a chemical reaction with a magnetically sensitive transient radical pair had a final yield dependent on the direction of an external magnetic field [6].
Cryptochrome, a light sensitive protein found in the bird’s eye, was found to form a radical pair when exposed to blue light [7]. The radical pair is created when blue light breaks a chemical bond, exciting the flavin adenin dinucleotide (FAD) and a triad of tryptophan amino acids (Trp) that consist the cryptochrome.
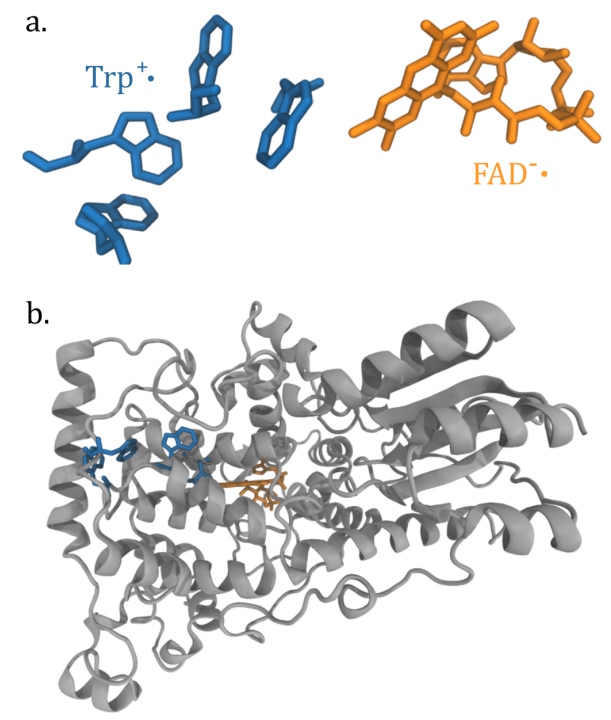
a)Visualization of the Trp and FAD molecules that form the radicals in cryptochrome.
b) Positioning of the radicals within cryptochrome protein.
- both electrons being spin up ( \( \uparrow _1 + \uparrow _2 \) )
- both electrons being spin down ( \( \downarrow _1 + \downarrow _2 \) )
- a superposition spin up and down, ( \( \uparrow _1 + \downarrow _2 \) ) + ( \( \downarrow _1 + \uparrow _2) \).